

The occlusiveness is because the oil will not allow water to evaporate from the surface of the skin. W/O emulsions tend to be immiscible in water, not water washable, will not absorb water, are occlusive, and may be “greasy.” This is primarily because oil is the external phase, and oil will repel any of the actions of water. Any semisolid character with w/o emulsions generally is attributable to a semisolid external phase. Many emulsions have internal phases that account for 40% – 50% of the total volume of the formulation. Stearic acid creams (sometimes called vanishing creams) are o/w emulsions and have a semisolid consistency but are only 15% internal phase volume.
Oil in water emulsion examples skin#
They are intended to be used in areas where the skin rubs against itself such as between the fingers, thighs, and under the arms.Įmulsions are also used as ointment bases and intravenously administered as part of parenteral nutrition therapy.

The term “lotion” is not an official term, but is most often used to describe fluid liquids intended for topical use.
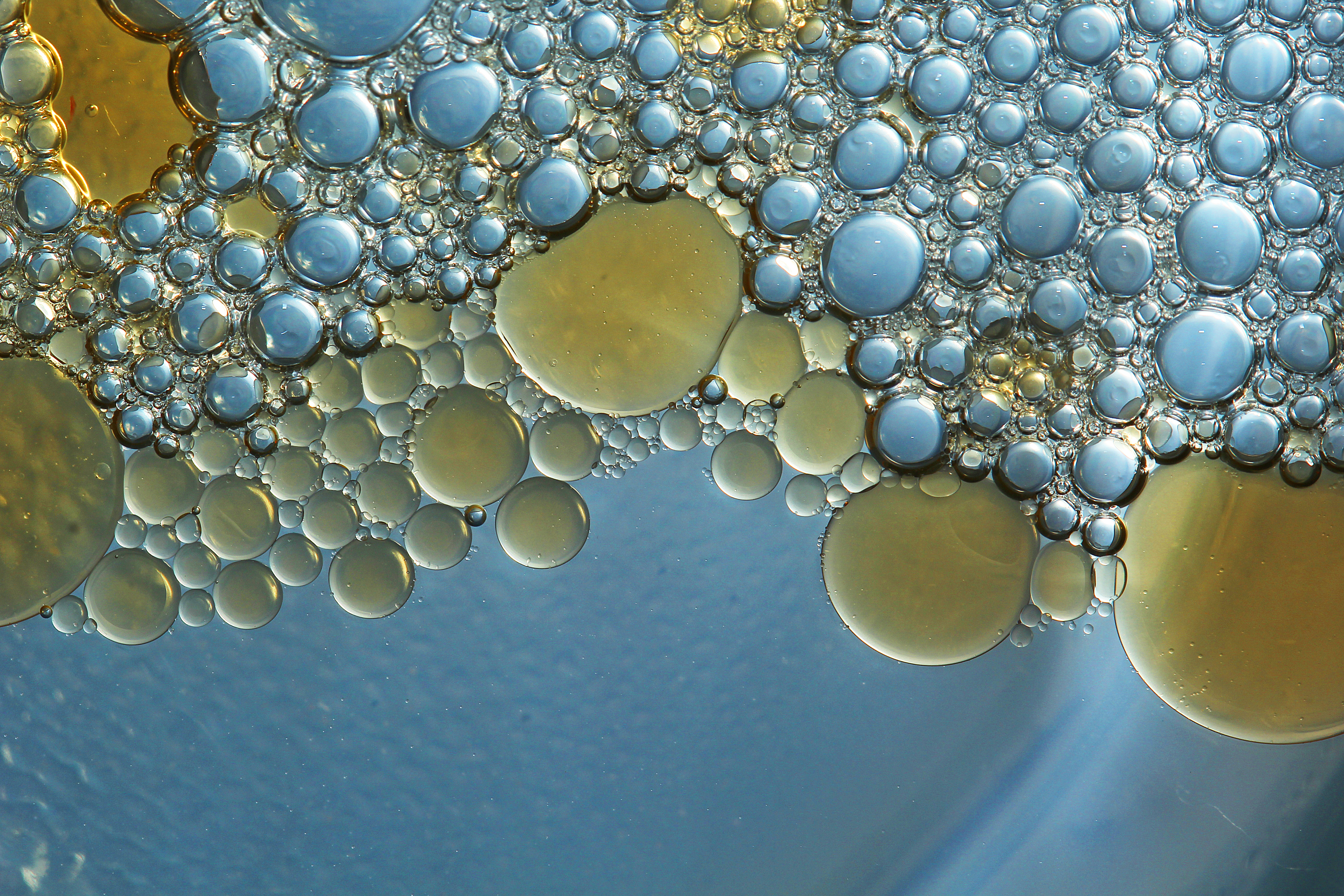
Emulsions are also the bases used in lotions, as are suspensions. They can be either o/w or w/o and are generally opaque, thick liquids or soft solids. Topical emulsions are creams which have emollient properties. More typically, emulsions are used for topical administration. But some times, emulsions are the formulation of choice to mask the taste of a very bitter drug or when the oral solubility or bioavailability of a drug is to be dramatically increased. Oral administration can be used, but patients generally object to the oily feel of emulsions in the mouth. W/O emulsions are generally formed if the aqueous phase constitutes < 45% of the total weight and an lipophilic emulsifier is used.Įmulsions are used in many routes of administration. Conversely, where water or aqueous solutions are dispersed in an oleaginous medium, the system is known as a water-in-oil (w/o) emulsion. An o/w emulsion is generally formed if the aqueous phase constitutes > 45% of the total weight, and a hydrophilic emulsifier is used. Where oils, petroleum hydrocarbons, and/or waxes are the dispersed phase, and water or an aqueous solution is the continuous phase, the system is called an oil-in-water (o/w) emulsion. The dispersed liquid is known as the internal or discontinuous phase, whereas the dispersion medium is known as the external or continuous phase. An emulsion is a thermodynamically unstable two-phase system consisting of at least two immiscible liquids, one of which is dispersed in the form of small droplets throughout the other, and an emulsifying agent.


 0 kommentar(er)
0 kommentar(er)
